The Risk Evaluation and Mitigation Strategy (REMS) for SPRAVATO (esketamine) is a program mandated by the U.S. Food and Drug Administration (FDA) to ensure the safe use of the medication due to its potential risks. The REMS program for SPRAVATO includes specific elements to mitigate these risks and enhance patient safety.
This program aims to minimize the potential risks associated with Spravato, particularly sedation, dissociation, and abuse or misuse. It’s essential to consult the most recent and official sources, including the medication’s prescribing information and the SPRAVATO REMS program materials, for the latest details.
Let’s delve into who can benefit from the Spravato REMS program and why it plays a vital role in the treatment journey.
Who Can Benefit from the REMS Program?
The Spravato REMS Program benefits a wide range of individuals, including:
Patients with Treatment-Resistant Depression:
- Individuals who have not responded adequately to at least two different antidepressant treatments.
- Offers a specialized and monitored approach for those facing treatment-resistant depression.
Healthcare Providers:
- Psychiatrists and mental health specialists play a pivotal role.
- Receive comprehensive guidelines and educational resources.
- Equipped to administer Spravato safely, elevating the standard of mental health care.
Pharmacists:
- Integral in ensuring proper distribution and dispensing of Spravato.
- Receive specific training through the program.
- Emphasis on adherence to safety protocols in handling and dispensing Spravato.
Caregivers and Support Systems:
- Acknowledges the role of caregivers in a patient’s mental health journey.
- Provides valuable information and resources to support systems.
- Fosters a collaborative approach to patient care, recognizing the importance of a holistic support system.
The Healthcare Ecosystem:
- Extends beyond individual roles to impact the broader healthcare system.
- Ensures standardized protocols are followed by all stakeholders.
- Contributes to a more structured and regulated approach to Spravato treatment.
- Elevates the overall integrity and reliability of the healthcare system.”
The Spravato REMS Program reaches across various roles in the healthcare landscape, creating a network of support, expertise, and adherence to safety standards. This collaborative effort is integral to the success of Spravato as a groundbreaking treatment for individuals dealing with treatment-resistant depression.
Eligibility Criteria: Who Qualifies for the SPRAVATO REMS Program?
The SPRAVATO REMS (Risk Evaluation and Mitigation Strategy) program is designed to ensure the safe use of SPRAVATO (esketamine) in individuals dealing with treatment-resistant depression (TRD). To qualify for the SPRAVATO REMS Program, individuals need to meet specific eligibility criteria, as mandated by the U.S. Food and Drug Administration (FDA).
The eligibility criteria for the REMS program for Spravato may include, but are not limited to, the following considerations:
Diagnosis:
- Individuals diagnosed with treatment-resistant depression (TRD) may be eligible for Spravato treatment under the REMS program. TRD is generally defined as a condition where individuals have not responded adequately to at least two different antidepressant treatments.
Prescribing Healthcare Provider:
- Healthcare providers who can prescribe Spravato within the REMS program typically include psychiatrists or other qualified mental health specialists. These providers must be certified and enrolled in the Spravato REMS program.
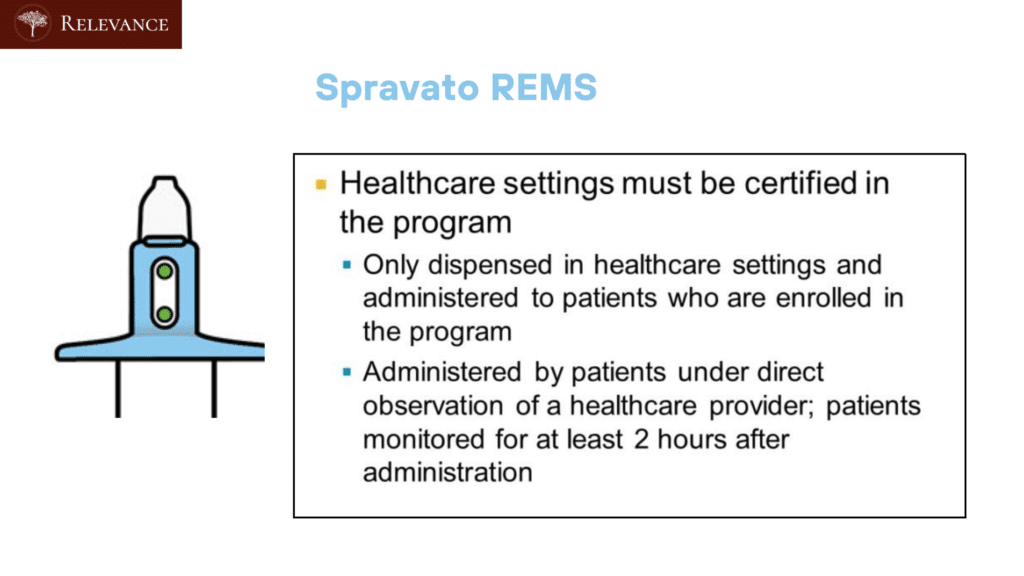
Administration Setting:
- Spravato is administered in a healthcare setting under the supervision of a healthcare professional. Individuals eligible for the REMS program would need to receive Spravato in an approved healthcare facility equipped to manage potential side effects.
Informed Consent:
- Individuals must provide informed consent before starting Spravato treatment. This involves understanding the potential risks, benefits, and requirements associated with the medication.
Monitoring and Compliance:
- Participation in the REMS program requires ongoing monitoring of the individual’s response to Spravato and compliance with the treatment plan. Regular check-ins with the prescribing healthcare provider are typically part of the program.
It’s essential to note that eligibility criteria may evolve, and the specific requirements for participation in the Spravato REMS program can be subject to change. Therefore, individuals considering Spravato treatment should consult with their healthcare provider for the most up-to-date and accurate information regarding eligibility and program requirements. Additionally, healthcare providers play a crucial role in assessing eligibility and guiding individuals through the necessary steps for participation in the REMS program.
Case Study: Enhancing Patient Safety through the Spravato REMS Program
John, a 38-year-old individual diagnosed with treatment-resistant depression (TRD), was exploring alternative treatment options after finding limited success with traditional antidepressants. His healthcare provider suggested considering Spravato (esketamine) and explained the importance of the Spravato REMS (Risk Evaluation and Mitigation Strategy) program for ensuring safe and effective treatment.
Background:
John’s history included multiple unsuccessful attempts with various antidepressant medications. His healthcare provider determined that Spravato might be a suitable option due to its unique mechanism of action and potential for rapid relief, especially for individuals with TRD.
Enrollment in the Spravato REMS Program:
Upon expressing interest in Spravato, John’s healthcare provider explained the REMS program’s significance and the necessity of enrollment. The REMS program aims to enhance patient safety by providing specific guidelines and monitoring procedures. John’s provider facilitated his enrollment by completing the required training and certification processes.
Eligibility Criteria:
To participate in the Spravato REMS program, John had to meet certain eligibility criteria, including:
- A diagnosis of treatment-resistant depression.
- Completion of informed consent, acknowledging an understanding of the risks and benefits associated with Spravato.
- Willingness to receive Spravato only in a certified healthcare setting under the supervision of a qualified healthcare professional.
Treatment Initiation:
Once enrolled in the REMS program, John and his healthcare provider worked together to develop a personalized treatment plan. The plan included details about the frequency and dosage of Spravato administration, as well as a schedule for monitoring sessions.
Outcome:
John responded positively to Spravato, experiencing a reduction in depressive symptoms. The close monitoring facilitated by the REMS program allowed for ongoing adjustments to optimize the treatment’s effectiveness while ensuring his safety.
This case study illustrates the role of the Spravato REMS program in promoting patient safety, informed decision-making, and personalized care for individuals with treatment-resistant depression. By adhering to the program’s guidelines, both patients and healthcare providers contribute to a collaborative and monitored treatment journey.
The Significance of REMS in SPRAVATO Treatment:
Understanding why the REMS (Risk Evaluation and Mitigation Strategy) is crucial for SPRAVATO treatment can be straightforward when broken down into key points:
Reducing Risks:
- The REMS program is like a safety net, identifying and lowering potential risks linked to using SPRAVATO. This makes sure that individuals undergoing treatment are protected from possible side effects.
Safe Administration:
- SPRAVATO is required to be given in a certified healthcare setting under the watchful eyes of healthcare professionals. This controlled setup makes certain that the medication is used safely, with immediate responses available for any unexpected reactions.
Expert Healthcare Providers:
- The REMS program insists on special training for healthcare providers involved in prescribing SPRAVATO. This training equips them to handle the unique aspects of SPRAVATO treatment, making sure patients get the best care.
Personalized Treatment Plans:
- SPRAVATO REMS focuses on creating treatment plans tailored to each individual. This means figuring out the right dosage and frequency based on factors like the patient’s response and medical history. Personalization helps make the treatment more effective.
Informed Decision-Making:
- Informed consent is a big part of the REMS program. It ensures that individuals get all the information they need about the risks and benefits of SPRAVATO. This way, patients can actively take part in deciding their treatment, giving them a sense of control.
Structured Monitoring and Adjustments:
- Regular check-ins during and after SPRAVATO sessions are a key part of the REMS program. This continuous evaluation allows healthcare providers to closely check how the patient is doing and make any needed changes to the treatment plan. This process optimizes the benefits while minimizing risks.
Following the Rules:
- The REMS program is not just a suggestion; it’s a rule from the U.S. FDA. This means everyone involved in SPRAVATO treatment, from healthcare providers to patients, follows a set of guidelines to ensure the medication is used properly and safely.
Staying Informed: The Importance of Consulting Official Sources:
Given the dynamic nature of healthcare regulations, it is imperative to stay informed. Consulting official and up-to-date sources, including the medication’s prescribing information and the SPRAVATO REMS program materials, ensures that both healthcare providers and patients are equipped with the latest and most accurate information.
The Final Say:
In our journey through mental health solutions, the Spravato REMS program is like a guardian angel, especially for those dealing with tough-to-treat depression.. It’s not just a set of rules; it’s a safety plan required by the FDA to make sure using Spravato is as safe as possible.
Understanding if you’re eligible is like opening a door to a better treatment experience. For people with tough depression, this program isn’t paperwork; it’s a chance at a brighter future. Saying “yes” to the program means saying “yes” to safer use, better-informed choices, and a treatment plan designed just for you.
To keep things clear, always check the most recent info from official sources. Look up the medication’s details and the Spravato REMS program materials for the latest, most accurate info.
Curious to learn more? Explore the ins and outs of the Spravato REMS Program here.